


dosage forms unit i dr.n damodharan professor and head department of pharmaceutics srm college of pharmacy
Carter Pharmaceutical Consulting Inc. is a Canadian company, providing consulting services in the area of solid oral dosage form development and manufacturing.
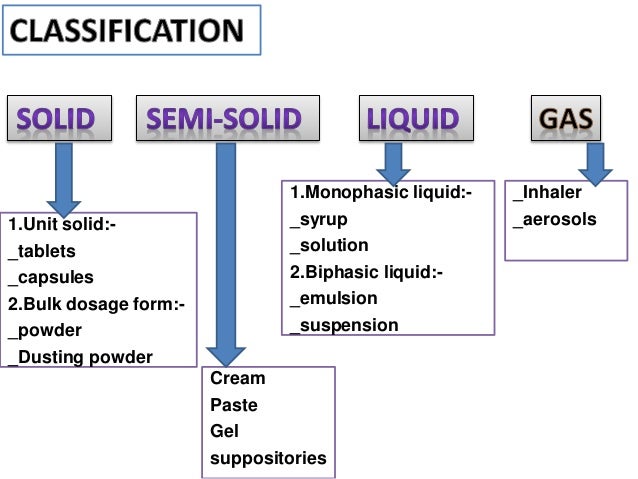
Expiration Dating of Unit-Dose Repackaged Solid Oral Dosage Form Drug Products Guidance for Industry . DRAFT GUIDANCE. This guidance document is being distributed for comment purposes only.

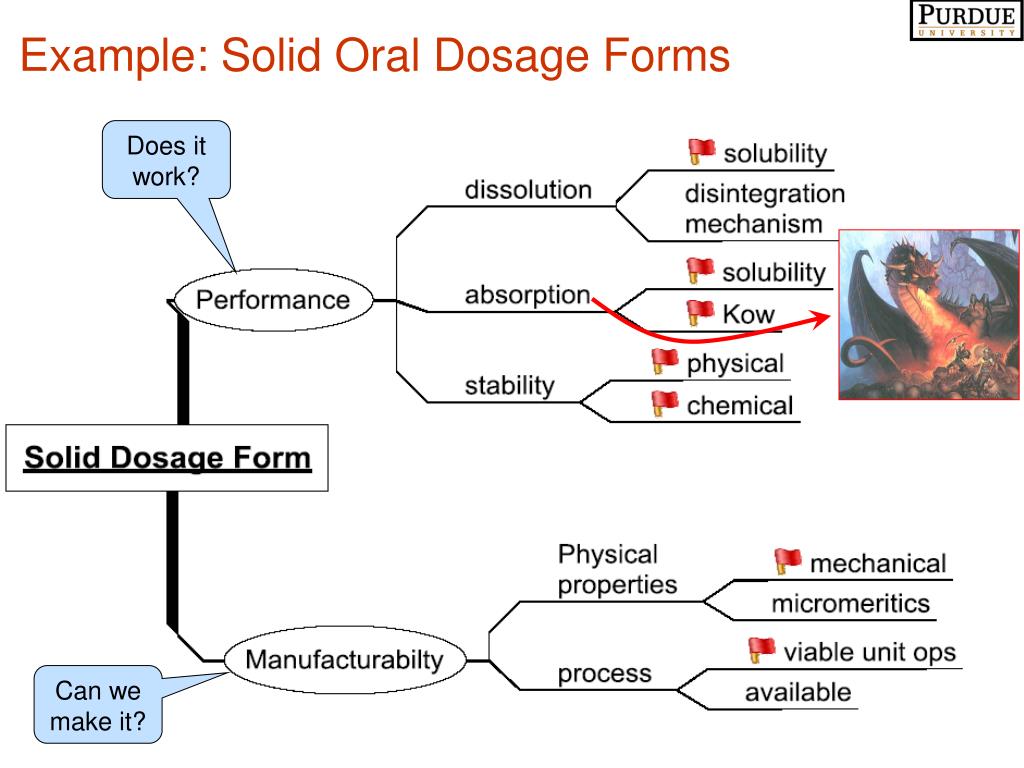
In the pharmaceutical industry, particle characterization of powder materials has become one of the crucial aspects in drug product development and quality control of solid oral dosage forms.
This article introduces the concepts of pooled variance and the central limit theorem, which are intended for establishing acceptance criteria for blend uniformity data of granular powder blends when a significant degree of sampling bias is involved.
Dave DiProspero, Co-Team Leader of the ISPE Baseline® Guide: Oral Solid Dosage Forms (Third Edition), offers insight about what brought on the update and what core takeaways you’ll get from the new edition of this Guide.
2 Section 1: Introduction This guideline describes the principles of procedures of bioequivalence studies for oral solid dosage forms that contains a different quantity of the active ingredient from an

the USP recommended ionic strength.5 For APIs that exhibit low solubilities in aqueous me-dia throughout the pH range, the addition of surfactants is recom-mended.
2 Section 1: Introduction This guideline describes the principles of procedures of bioequivalence studies for post-approval changes in the components and composition of oral solid dosage forms
1005598 FNL . Waiver of In Vivo Bioavailability and Bioequivalence Studies for Immediate-Release Solid Oral Dosage Forms Based on …
